House legislators lobby to exclude 12-year data exclusivity period from free trade agreement
Patent Docs | August 11, 2011
House legislators lobby to exclude 12-year data exclusivity period from free trade agreement
By Donald Zuhn —
Last week, a group of seven House Democrats, led by Rep. Henry Waxman (D-CA), sought to have the 12-year data exclusivity period provided by the biosimilar approval pathway of the Patient Protection and Affordable Care Act (PPACA) excluded from the Trans-Pacific Partnership (TPP) agreement. According to a report in The Hill, the legislators sent a letter to President Obama, asking him to exclude the 12-year period from the TTP’s intellectual property provisions.
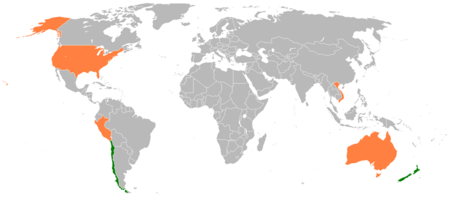
The TTP, or Trans-Pacific Strategic Economic Partnership Agreement, is a multilateral free trade agreement currently being negotiated by Australia, Brunei, Chile, Malaysia, New Zealand, Peru, Singapore, the United States, and Vietnam (Canada, Japan, the Philippines, South Korea, and Taiwan have also expressed interest in participating in the agreement). The seventh round of TTP negotiations was held in Ho Chi Minh City, Vietnam, in June, and two more rounds of negotiations are scheduled to take place in September (in the United States) and in October (in Peru). The nine TPP members have set a goal of reaching the outlines of an agreement by November of this year.
In the letter sent to the President, the House members stated that "[w]ere the TPP to ultimately contain a 12 year biologics exclusivity provision, it would impede the ability of Congress to achieve the Administration’s proposed 7 year change without running afoul of U.S. trade obligations." The legislators added that "[w]e see no reason for the United States to agree to such a provision, much less propose it." Prior to passage of the PPACA in March of last year, Rep. Waxman had introduced biosimilar legislation in the House that would, like the Hatch-Waxman regulatory pathway for small molecule generic drugs, provide 5 years of exclusivity for generic biologics (see "Waxman Introduces Follow-on Biologics Bill"). In addition, the Obama Administration, via the Office of Management and Budget (OMB), released a letter in June of 2009 that advocated for a 7 year exclusivity period (see "White House Recommends 7-Year Data Exclusivity Period for Follow-on Biologics"). Earlier this year, the President unveiled his 2012 budget proposal, which if approved unchanged, would trim the data exclusivity provision of the PPACA from 12 years to 7 years (see "President’s Budget Proposal Increases Funding for Basic Research But Seeks to "Trim" Data Exclusivity Period and Pay-for-Delay Agreements").





