A Trade Agreement’s Impact On Access To Generic Drugs
The Central America Free Trade Agreement
has kept some generic drugs from Guatemala
even though they’re available in the United States.
by Ellen R. Shaffer and Joseph E. Brenner
ABSTRACT:
Millions of people lack access to affordable medicines. The intellectual property rules in the Central America Free Trade Agreement (CAFTA) provide pharmaceutical companies with monopoly protections that allow them to market some drugs without competition by less costly generics. We examined availability of certain drugs in Guatemala and found that CAFTA intellectual property rules reduced access to some generic drugs already on the market and delayed new entry of other generics. Some drugs protected from competition in Guatemala will become open for generic competition in the United States before generic versions will be legally available in Guatemala. [Health Affairs 28, no. 5 (2009): w957-w968 (published online 25 August 2009; 10.1377/hlthaff.28.5.w957)]
The Central America Free Trade Agreement (CAFTA) covers the United States, five Central American countries (Costa Rica, El Salvador, Guatemala, Honduras, and Nicaragua), and the Dominican Republic. [1] Its rules protect the products and processes of brand-name pharmaceutical companies—intellectual property—from competition by generic companies. Generic competition can lower drug prices. CAFTA’s rules on intellectual property provide stronger monopoly protections than in existing U.S. law or the World Trade Organization’s multilateral Agreement on Trade-Related Aspects of Intellectual Property (TRIPS). [2] The World Health Organization and others have expressed concerns that these "TRIPS-Plus" rules will further delay competition from generic companies and will have the most serious consequences in lower-income countries, where price is an important factor in access. [3]
We examined intellectual property provisions of CAFTA and their effects on access to lower-price medicines, including generics, in Guatemala—a low-income country that also has a domestic generic drug industry. [4] This paper focuses on one key TRIPS-Plus rule, known as data exclusivity, which provides relatively quick access to monopoly protection, and related higher prices.> [5]
In 2007 the U.S. Congress took steps to reduce the extent of some TRIPS-Plus rules, including data exclusivity, in an agreement with Peru. [6] Nevertheless, the rules remain in place in CAFTA and other agreements, some are included in ongoing negotiations, and all may resurface in the future. [7] This review therefore provides a useful case example of issues that other countries may confront in implementing CAFTA and similar agreements, and it offers policy recommendations.
Study Data And Methods
To determine whether CAFTA intellectual property rules are limiting access to generic and lower-price medicines in Guatemala, we reviewed relevant provisions in Guatemalan law, TRIPS, and CAFTA. We collected information about public and private drug purchasing systems in Guatemala, including prices where available. We obtained lists of drugs available through various public-sector health programs described below and in the private sector, during site visits to Guatemala in 2006 and 2007 and via correspondence with key informants and online sources posted by the Ministerio de Salud Pública y Asistencia Social, or Ministry of Health. [8] We compared drugs that have gained monopoly protection through CAFTA intellectual property rules on data exclusivity (referred to as "data-protected drugs") and patents, with drugs removed from the market or denied entry because of conflicts with these rules.
To determine the influence of data exclusivity on pricing, we used the Ministry of Health’s Open Contract prices from 2005 and 2007 as the best available indicators of relative prices. To further establish the price implications of introducing data-protected drugs to the market and conferring data-protected status to drugs already offered in Guatemala, we identified several data-protected drugs used for conditions that are common causes of morbidity and mortality, and also for HIV/ AIDS—the latter because the virus often develops resistance to first-line drugs, requiring the use of newer medicines. We compared these drug prices with those of therapeutically equivalent brand-name and generic medicines that are not data-protected and that were already offered on the Open Contract lists in 2005 and 2007. Finally, we looked at when intellectual property protections expired in the United States for the medicines that are data-protected in Guatemala.
In Guatemala we interviewed governmental and private officials in the health sector and individuals from business and academia regarding the structure of the drug distribution system and possible and actual effects of CAFTA’s intellectual property rules. [9]
TRIPS, CAFTA, Patents, And Data Exclusivity
In the past, originator drug companies have generally not invested the time and expense to obtain patents on most of their drugs in Guatemala, as in most low- <->income countries, where revenues are likely to be low and little competition is expected. This is changing because of CAFTA and TRIPS.
Among its other provisions, the TRIPS Agreement requires all 153 World Trade Organization member nations to recognize product and process patents for twenty years. [10] Guatemala did so in 1999, with the Law on Industrial Property, Accord 712.99. [11] More than 300 pharmaceutical products and processes in Guatemala are patented, and 246 patent applications were filed in the first ten months of 2007. [12] Most of the substances for which applications were filed are not yet associated with drugs on the market. A marketed drug can embody patents on numerous molecules and processes. Although no public list describes which products ultimately will be covered by these patents, a representative from the local generic industry association, ASINFARGUA, reported that the list of products has been referred to as the basis for denying marketing rights to generics. [13]
Data exclusivity is a TRIPS-Plus rule that inserts an administrative barrier to the marketing of generic drugs even when there is no patent in place. [14] Brand-name drugs must be proven safe and effective through clinical studies involving human subjects. To bring their drugs to market, generic drug companies must demonstrate only that their drugs are bioequivalent to brand-name drugs—that is, they work the same way in the body. Generic drug manufacturers establish safety and efficacy by referring to the results of the clinical trial data already produced by the drugs’ brand-name equivalents. However, generic companies are prohibited from using or referring to the originator’s clinical trial data for drugs during the period of time that they are data-protected.
Under CAFTA and related domestic law, Guatemala authorizes brand-name drugs to be data-protected for either five or fifteen years. [15] Originator companies can select which medicines they submit to the Guatemalan drug regulatory agency to be listed as data-protected. This is a simpler process for drug companies than getting a patent, and it offers the same monopoly marketing rights, although for a shorter term. The result is that generic equivalents of data-protected drugs are effectively barred from entering the market in Guatemala for several years.
Background On Guatemala’s System
Guatemalan intellectual property laws. Intellectual property rules have been a contentious legislative issue in Guatemala since the late 1990s. Key provisions of the initial 1999 Law on Industrial Property, Accord 712.99, have been amended almost every year since it was adopted. The rules on data exclusivity were changed on every occasion. Legislators alternatively enacted data exclusivity in 2000 to run for a fifteen-year term on each covered drug, repealed it entirely in 2002, reenacted it to provide a five-year term in 2003, and repealed it again in 2004, before codifying it with a five-year term after the approval of CAFTA in 2005. [16] The United States threatened that Congress would not approve CAFTA unless Guatemala adopted laws that were harmonious with CAFTA on data exclusivity and other matters, prompting a letter of protest from some members of Congress. [17] The United States is Guatemala’s most important trading partner, acting as the market for 36 percent of Guatemalan exports and the source of 40 percent of its imports. [18]
CAFTA passed in Guatemala’s Congress in March 2005 and in the U.S. Congress—by two votes—in July 2005. It specifically states that trade agreements will prevail over the relevant domestic laws in the event of conflicts. [19]
Within Guatemala, the ministry’s Department for the Regulation and Control of Pharmaceuticals and Related Products regulates the registration of pharmaceuticals. Registration lasts for five years, after which it must be renewed. Some companies have been denied renewals because of intellectual property rules. [20] Fifty-five products were registered for five years of data exclusivity as of February 2008. [21] In addition, Guatemala continues to recognize data exclusivity for fifteen years for the twenty-two drugs registered while the relevant law requiring that period was in effect. [22] For our study, we selected protected drugs that are important in treating the most common health conditions: hypertension (Ventavis), cancer (Fludara, Aloxi, Emend, Erbitux), pneumonia (Invanz), diabetes (Lantus), and cardiac disease and stroke (Crestor). [23] We also looked at drugs for HIV/AIDS, as described below, and the one contraceptive (Yasmin) and two vaccines (Rotarix and Rotateq) on the list of data-protected drugs.
The Guatemalan drug purchasing systems. The public sector provides outpatient and hospital services and medicines through the Ministry of Health, which covers about 70 percent of the population; the better-funded Social Security system (Instituto Guatemalteco de Seguridad Social, or IGSS), which provides workplace-based coverage for about 10 percent of the population; and the Ministry of Defense, which serves the military. [24]
The Ministry of Health coordinates drug purchasing for the public sector. It identifies the categories and quantities of medicines it expects to use in the coming year and invites suppliers to submit bids to procure these medicines. Suppliers and prices are established through an open, public bidding process known as Contrato Abierto (Open Contract).
All bids are opened by a panel at a public meeting where up to eight of the lowest bids in each therapeutic category are selected. Ministry of Health-sponsored hospitals and clinics, as well as the IGSS, procure their drugs through these vendors. They are usually limited to ordering three-month supplies of these medicines. There are two important exceptions. If a drug of interest is not on the Open Contract list, public purchasers must seek other sources. Also, some suppliers may elect not to fulfill their contracts, for whatever reason. In these cases, both price and supplier are unknown. In 2007 there were about 730 medicines on the Open Contract list.
The IGSS generates a separate list of 630 drugs, many of which overlap with the Ministry of Health-coordinated Open Contract list. In addition, the Ministry of Health sponsors the Drug Access Program (Programa de Acesibilidad a Medicamentos, or PROAM), which offers about 100 basic drugs at reduced prices in the most marginalized communities. [25]
Study Findings
Effects of data-exclusivity rules. We found that CAFTA’s data exclusivity and patent rules as implemented in Guatemala through domestic law and regulation are limiting access to some generic drugs that are less costly substitutes for newly protected brand-name drugs. Some data-protected medicines are available through public-sector purchasers. A number of the protected drugs will become open for generic competition in the United States, where they were first launched, before generic versions will be legally available in Guatemala. [26] For example, clopidogrel bisulfate (Plavix), used to treat myocardial infarction, both is patented and has fifteen-year data exclusivity in Guatemala. [27] Four drug companies that formerly sold registered generic versions of clopidogrel bisulfate in Guatemala have had that registration revoked: Roemmers S.A. (Uruguay), Piersan (Guatemala), Panalab S.A. (Argentina), and Biocross (Guatemala). [28] The result is reduced competition.
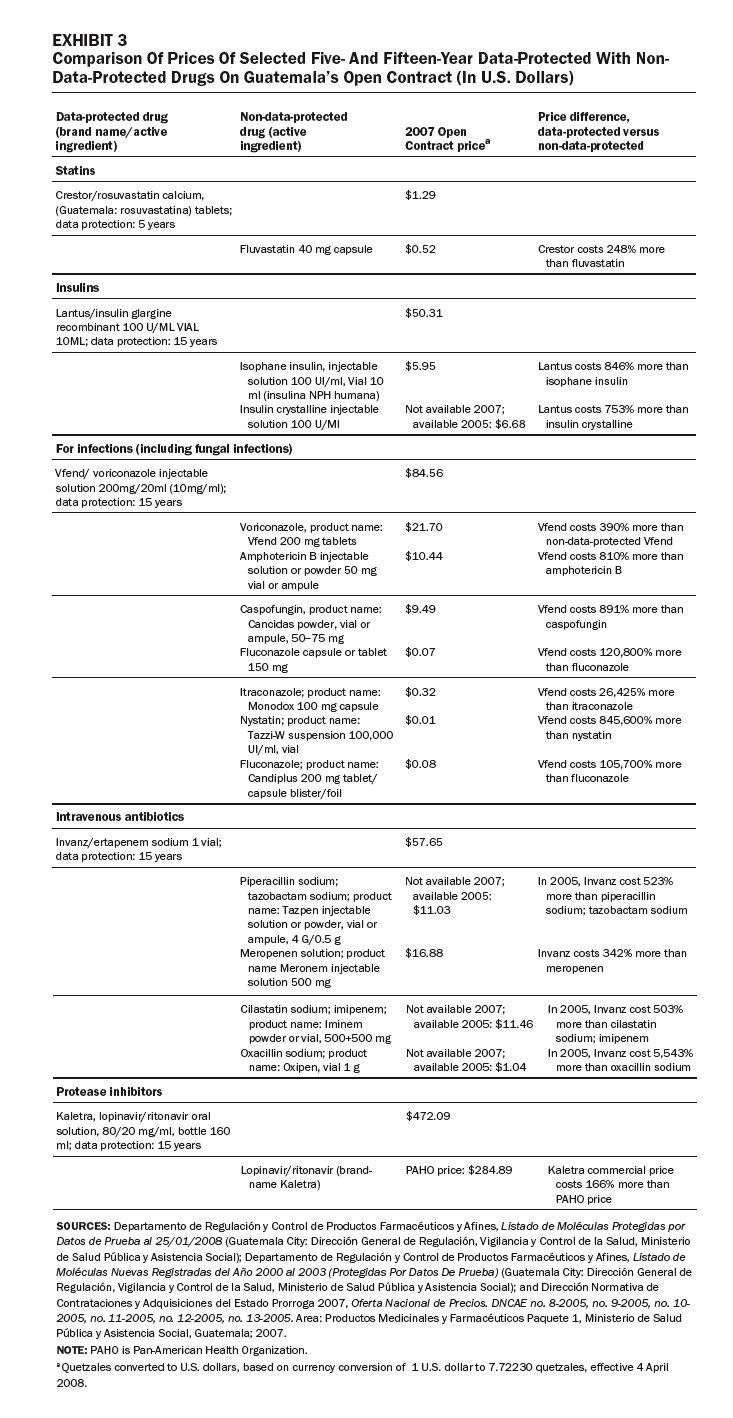
In other cases, some generic competitors have been denied entry to market in Guatemala because brand-name drugs are data-protected, or the drugs or their components are patented (Exhibits 1 and 2). These drugs are used to treat major causes of mortality and morbidity in Guatemala. Our review of the purchasing lists for the Ministry of Health, IGSS, and the Drug Access program identified eight data-protected brand-name drugs offered by public-sector purchasers.
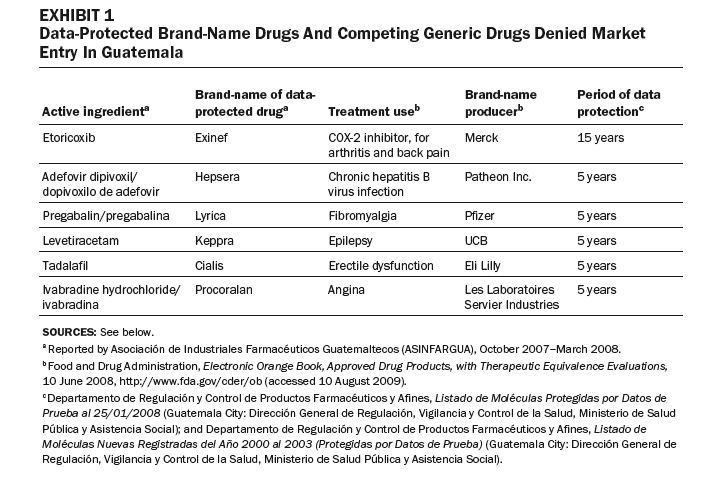
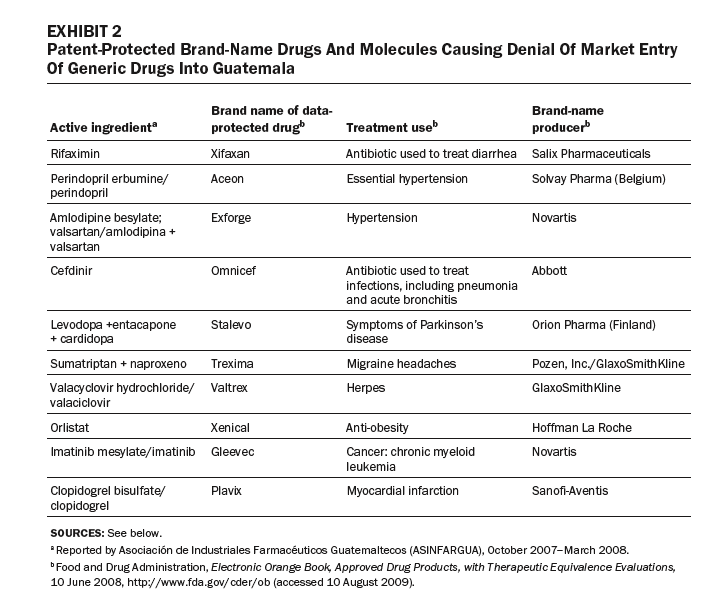
Exhibit 3 compares prices for a sample of five- and fifteen-year data-protected drugs with non-data-protected drugs listed on the Open Contract: statins, insulins, anti-infectives, antibiotics, and protease inhibitors. In each case, the data-protected drugs are much more expensive than nonprotected drugs in the same therapeutic class. For example, the insulin Lantus costs 846 percent more than isophane insulin; the antifungal Vfend costs 810 percent more than the non-data-protected amphotericin B; and the intravenous antibiotic Invanz costs 342 percent more than the non-data-protected meropenem (Meronem).
The price and supply of drugs for HIV/AIDS have been contentious in Guatemala. Protease inhibitors are key second-line drugs, used as people develop immunity to first-line treatments. Transnational drug companies have listed five protease inhibitors as being data-protected. As a result, prices of these drugs remain high, straining public-sector budgets. The case of lopinavir/ritonavir (Kaletra), a protease inhibitor produced by Abbott, is a good example. Registered on 29 November 2005, Kaletra was granted data exclusivity retroactively to 2000; it expires in 2015. [29] A generic version was registered as lopinavir/ritonavir (PF-33703) by Okasa Pharma Pvt. Ltd. (India) on 23 August 2004—earlier than Kaletra was registered and data-protected. Only Kaletra, the data-protected drug, was listed on Guatemala’s Open Contract. [30]
Efforts to obtain lower prices. Guatemala’s Ministry of Health has attempted several strategies to bring down the prices of needed medicines. The Pan American Health Organization has offered medicines at lower prices. [31] Decree 16-2003 in Guatemala permits waiving import taxes on AIDS drugs. This law is the subject of a preliminary trade charge known as a Special 301 complaint to the U.S. Trade Representative by the Pharmaceutical Research and Manufacturers of America. [32]
Some domestic laws make efforts to contain prices more difficult. For example, a 2007 law, 66-2007, limits the government to paying for no more than 20 percent of a given order at one time. Such financing or currency controls may further complicate the Ministry of Health’s ability to purchase through current suppliers.
Some forty-two drugs that are newly patented or covered by data exclusivity in Guatemala had already lost or will lose their patent or data exclusivity, or both, in the United States while remaining restricted in Guatemala. [33] This means that lower-price generic substitutes can be sold in the United States while they remain barred from the market in Guatemala. These include the following. Five-year data-protected drugs: iloprost; entecavir; levetiracetam; nesiritide; and palonosetron. [34] Fifteen-year data-protected drugs: fludarabine phosphate; bimatoprost; eletriptan hydrobromide; insulin glargine recombinant; and ertapenum sodium. [35]
Discussion And Recommendations
Our study suggests that CAFTA’s intellectual property rules on data exclusivity and patents are responsible for the removal of several lower-cost generic drugs from the market in Guatemala and for the denial of entry to a number of others. Our findings on prices are limited, because we were able to examine only published prices for pharmaceuticals purchased by the public sector. Because many Guatemalans are poor and depend on the public sector, these data are important. However, prices could be either higher or lower in the private sector. Further efforts to collect empirical data on the effects of particular TRIPS-Plus provisions are warranted.
The U.S. Congress removed the data-exclusivity provision of the trade agreement with Peru in May 2007, recognizing potentially negative consequences for lower-income countries. [36] As an initial step, the U.S. Trade Representative and the Department of Commerce should extend this recognition to all CAFTA countries. It should assert its intention not to implement the data-exclusivity provisions of CAFTA and should proactively cooperate with Central American governments that take action not to implement these provisions. A more limited reform proposal, generated prior to the Peru policy, would require generic producers to pay a small sum for the use of pharmaceutical test data, rather than the present system of establishing exclusive rights to protect investments in the data. [37]
More broadly, the United States should support Guatemala in exploring ways to obtain lower-price drugs from donors and on the market. In its trade objectives, the Obama administration and Congress should aim for a fairer balance between promoting access to medicines and long-term incentives for drug innovation, particularly in developing countries. The World Health Organization has endorsed a comprehensive set of proposals, which include reinvigorating local production to stimulate technology transfer that would initiate research and development capability in developing countries. [38] The World Health Organization has also endorsed replacing intellectual property regimes with public health approaches, such as prize funds to reward research and development, thereby delinking incentives for research and development from drug prices. [39] Proposals from the pharmaceutical industry include voluntary measures to make selected products available at lower prices in developing countries, and also to sponsor unrestricted patent pools available to researchers exclusively for neglected tropical diseases. [40] Ongoing international consideration should assess the best prospects for benefiting health.
The authors gratefully acknowledge financial support for this work from the Moriah Fund and Oxfam America. They thank the editors and anonymous reviewers for this journal and the many colleagues who have assisted with this work.. These include Mariel Castro, project director in 2007; Alejandro Ceron and Jennifer Crowe, on-site translators and advisers; and Luis Velasquez, Bessie Orozco, Claudia Herrarte, Juanita Mejia Rodriguez in Guatemala. For review of drafts and advice on methods, they thank Stephanie Burgos, Rohit Malpani, and Gawain Kripke, Oxfam America; Cesar Vieira; Nuria Homedes and Antonio Ugalde; and Arlene Ash. For assessments of medicines, they thank Sara Levin and Josh Rolnick. They also thank Kristen Smith, former CPATH Fellow, and Lily Walkover, CPATH outreach coordinator.
Ellen Shaffer (ershaffer@cpath.org) and Joseph Brenner are codirectors of the Center for Policy Analysis on Trade and Health (CPATH) in San Francisco,California.
Read related articles
DOI: 10.1377/hlthaff.28.5.w957
©2009 Project HOPE–The People-to-People Health Foundation, Inc.
Footnotes:
[1] United States Trade Representative, "CAFTA Final Text," 16 June 2009,
http://www.ustr.gov/tradeagreements/free-trade-agreements/cafta-dr-dominican-republic-central-america-fta/final-text (accessed 10 August 2009). These countries signed CAFTA in August 2004. The agreement entered into force in Guatemala on 1 July 2006. Guatemala is still drafting and implementing related legislation.
[2] 21 US Code 355(c)(E)(ii) limits data exclusivity in the United States to five years or less. TRIPS 39.3 allows data exclusivity only to protect against unfair commercial use and to protect new chemical entities. CAFTA 15.10 gives applicants (for example, pharmaceutical companies) the right to five years or more of data exclusivity, at the discretion of the applicant, and for conditions other than to protect against unfair commercial use or for the presentation of a new chemical entity. (Footnote 15 in CAFTA Article 15 preserves the right of the United States to maintain a shorter term for data exclusivity but does not offer this right to any other signatory country that did not already have a shorter term codified in law.)
[3] World Health Organization, Public Health, Innovation, and Intellectual Property Rights: Report of the Commission on Intellectual Property Rights, Innovation, and Public Health (Geneva: WHO, April 2006).
[4] A.M. Solares, Drugs and Pharmaceuticals: Vitamins/Nutritional Supplements, Industry Sector Analysis: Guatemala, 17 March 2003,
http://strategis.ic.gc.ca/eic/site/imr-ri.nsf/eng/gr112076.html (accessed 18 August 2009).
[5] CAFTA 15.101(a) states, "If a Party requires, as a condition of approving the marketing of a new pharmaceutical or agricultural chemical product, the submission of undisclosed data concerning safety or efficacy, the Party shall not permit third persons, without the consent of the person who provided the information, to market a product on the basis of (1) the information, or (2) the approval granted to the person who submitted the information for at least five years for pharmaceutical products." This gives companies ("the person who provided the information") the right to five years or more of exclusive use of their clinical trial data, during which time other companies ("third persons") cannot use or refer to those data to support their applications for marketing. CAFTA 15.10.1(b) confers these same rights in the case of products already marketed in another country. TRIPS 39.3 allows data exclusivity only to protect against unfair commercial use and to protect new chemical entities. CAFTA leaves the use of these data to the discretion of the originator company and does not limit the grounds for data protection to protecting against unfair commercial use or for the presentation of a new chemical entity.
[6] Office of the United States Trade Representative, "Trade Facts, Bipartisan Agreement on Trade Policy: Intellectual Property Provisions," May 2007, http://www.ustr.gov/sites/default/files/uploads/factsheets/2007/asset_upload_file945_11283.pdf (accessed 10 August 2009).
[7] Data exclusivity is included in existing U.S. trade agreements with Chile, Singapore, Jordan, Morocco, Bahrain, and Australia, and in the agreement with South Korea that is pending congressional action.
[8] These sources are available in an appendix, online at http://content.healthaffairs.org/cgi/content/full/ hlthaff.28.5.w957/DC2.
[9] This paper summarizes key findings from a more extensive report and document library to be posted online at http://www.cpath.org. In Guatemala we interviewed a deputy minister of health, the regional representative of the Pan American Health Organization who formerly directed the Guatemalan Department for the Regulation and Control of Pharmaceuticals, the director of the generic pharmaceutical association (ASINFARGUA), a representative of a generic drug company (Biocross), a professor in the pharmacy department of the national university, and the director of the government program on AIDS medicines.
[10] Implementation can be delayed for some low-income countries.
[11] Departamento de Regulaci—n y Control de Productos Farmacéuticos y Afines, Leyes y Reglamentos, Accord 712.99, Guatemala (Guatemala City: Direcci—n General de Regulaci—n, Vigilancia y Control de la Salud, Ministerio de Salud Pública y Asistencia Social, 28 November 2008).
[12] DRCPFA, Patentes Farmacéuticas (Guatemala City: DGRVCS, MSPAS).
[13] Claudia Herrate, on behalf of ASINFARGUA, personal communication, 26 November 2007.
[14] For a discussion of the role of TRIPS-Plus measures, see V.B. Kelly and K. Lee, "TRIPS, the Doha Declaration and Paragraph 6 Decision: What Are the Remaining Steps for Protecting Access to Medicines?" Globalization and Health 3, no. 3 (2007). In higher-income countries like the United States, where drugs are commonly patented, data-exclusivity rules may also apply. They usually run simultaneously with patents, for a shorter term, and they have a different effect on price and access.
[15] The term of data exclusivity depends on whether the drug was listed during the years when the law offered a fifteen-year term, or in later years when the law offered five years.
[16] A summary of the changes follows. In 2000, Decree 57-2000 authorized brand-name companies to register their products for data exclusivity for fifteen years. A list of twenty-two data-protected drugs was created. In 2002, Decree 76-2002 eliminated data exclusivity for any time period. In 2003, Decree 9-2003 repealed Decree 76-2002 and implemented five years of data exclusivity. The U.S. Department of Commerce’s Special 301 Watchlist is a prelude to possible trade sanctions. In 2003 the Special 301 Watchlist noted unfavorably Decree 76-2002 and encouraged the ongoing implementation of Decree 9-2003. Office of the U.S. Trade Representative, 2003 Special 301 Report (Washington: Executive Office of the President, 2003), 22. Decree 9-2003 also stated that the protected data "must require considerable effort to produce," an attempt to limit the frivolous listing of medicines. In 2004, Decree 34-04 repealed Decree 9-2003, again eliminating data exclusivity. As CAFTA was being negotiated in 2005, Decree 30-2005 repealed Decree 34-04 and reinstated a five-year period for data exclusivity. It states that trade agreements will prevail over this law in the event of conflicts.
[17] Embassy of the United States, Guatemala, "CAFTA, Data Protection, and Generic Drugs, Fact Sheet Generics," 10 January 2005, http://guatemala.usembassy.gov/factsheetcaftagenerics.html (accessed 10 August 2009). The document states: "This law [34-2004] gives the U.S. Congress the impression that Guatemala is not serious about complying with commitments it made in the CAFTA. This could result in CAFTA not being ratified by the U.S. Congress, where a close vote is expected." See also H.L. Solis, H.A. Waxman, and C.B. Rangel, Letter to the Honorable Robert B. Zoellick, U.S. Congress, 26 January 2005, http://www.cpath.org/sitebuildercontent/sitebuilderfiles/congressguatemalatest-data-secrecy-letter1-05.pdf (accessed 18 August 2009).
[18] Trade Compliance Center, U.S. Department of Commerce, Press release, 18 January 2002,
http://tcc.export.gov/Country_Market_Research/All_Research_Reports/exp_005742.asp (accessed 10 August 2009).
[19] The implementing legislation mitigated the problem somewhat by requiring that an originator company must apply for approval in Guatemala within five years of a drug’s initial approval in another country. This means, for example, that if the brand-name company introduces its drug in Guatemala six years after its launch in the United States, data exclusivity rules would not apply.
[20] ASINFARGUA, personal communication, 21 November 2007.
[21] DRCPFA, Listado de Moléculas Protegidas por Datos de Prueba al 25/01/2008 (Guatemala City: DGRVCS, MSPAS, 25 January 2008).
[22] DRCFPA, Listado de Moléculas Nuevas Registradas del Año 2000 al 2003 (Protegidas por Datos de Prueba) (Guatemala City: DGRVCS, MSPAS).
[23] Comisi—n para el Mejoramiento de las Estadísticas y Análisis de Genero en Salud, Perfil de Salud de Mujeres y Hombres en Guatemala (Guatemala City: Ministerio de Salud Pública, 2005).
[24] Ibid.
[25] Pan American Health Organization, Health Systems Profile Guatemala, 3d. ed., February 2007, p. 41,
http://www.lachealthsys.org/index.php?option=com_docman&task=doc_download&gid=169&Itemid= (accessed 18 August 2009); and Programa de Accesibilidad de Medicamentos, "Listados Básicos," http://www.proam.gob.gt/listados.html (accessed 18 August 2009).
[26] Food and Drug Administration, Electronic Orange Book, Approved Drug Products with Therapeutic Equivalence Evaluations, 10 June 2009, http://www.fda.gov/cder/ob (accessed 10 August 2009); DRCPFA, Listado de Moléculas Protegidas; and DRCPFA, Listado de Moléculas Nuevas Registradas del A-o 2000 al 2003.
[27] Plavix is certainly patented in Guatemala. Its data-exclusivity status is less clear. In 2007 the Department of Regulation reported that Plavix was supposed to be listed as one of the twenty-two drugs protected for fifteen years while the original decree was in effect. The department reversed this determination at some point. Its present status is unclear.
[28] Reported by key informant in Guatemala based on interviews with Drug Regulatory Agency. There is at least one other company that has registered clopidogrel (Laboratorias Stein in Costa Rica), but it is not known whether its registration was revoked.
[29] MSPAS, Dirección General de Regulaci—n, Vigilancia y Control de la Salud, Departamento de Regulaci—n y Control de Productos Farmaceùticos y Afines, Productos Farmaceùticos con Registro Sanitario Autorizado Vigente al 28/09/2007 (Guatemala City: MSPAS, 2007). Currently, registration for drugs in Guatemala is for a five-year period. The date listed on the Ministry of Health document is for the date that registration expires. The registration date was extrapolated based on the date of expiry. See DRCPFA, Listado de Moléculas Nuevas Registradas del A-o 2000 al 2003.
[30] Ministerio de Salud Pública y Asistencia Social, Dirección Normativa de Contrataciones y Adquisiciones del Estado Prorroga 2007, Oferta Nacional de Precios DNCAE no. 10-2005, Area: Productos Medicinales y Farmaceuticos Paquete 1 (Guatemala City: Ministerio de Salud Pública, 2007).
[31] A schedule provided by PAHO lists forty-five medicines that either are not available through the open contract at the prices accepted from bidders or are less expensive through PAHO than the Open Contract price.
[32] USTR, Pharmaceutical Research and Manufacturers of America (PhRMA) Special 301 Submission 2008 (Washington: USTR, 2008), 247-248.
[33] CAFTA applies both to the United States and to Guatemala. As described in Note 2, CAFTA allows the United States to maintain its pre-existing law that permits data-exclusivity terms for less than five years. The term may also expire sooner in the United States if it runs simultaneously with a patent term, which is longer.
[34] FDA, Electronic Orange Book; and DRCPFA, Listado de Moléculas Protegidas.
[35] Ibid.; and DRCPFA, Listado de Moléculas Nuevas Registradas del Año 2000 al 2003.
[36] USTR, "Trade Facts."
[37] R. Weissman,. "Public Health-Friendly Options for Protecting Pharmaceutical Registration Data," International Journal of Intellectual Property Management 1, nos. 1/2 (2006): 113-130.
[38] World Health Assembly, "Global Strategy and Plan of Action on Public Health, Innovation, and Intellectual Property," WHA61.21 Agenda item 11.6 (Geneva: WHA, 24 May 2008). In the Annex, Elements 2 and 3 discuss improving research and development capacity and incentive schemes for health-related innovation. Element 4 addresses technology transfer. See also WHO, Public Health, Innovation, and Intellectual Property Rights.
[39] WHA, "Global Strategy and Plan of Action."
[40] S. Dentzer, "Eliminating Neglected Diseases in Poor Countries: A Conversation with Andrew Witty," Health Affairs 28, no. 3 (2009): w411-w416 (published online 19 March 2009; 10.1377/hlthaff.28.3.w411).