
IP Watch | 1 December 2017
EU-Mercosur FTA puts at risk access to medicines in Brazil, new impact assessment study finds
Authors:
- Marcela Fogaça Vieira – human rights and IP lawyer, master in Public Health, consultant Shuttleworth Foundation
- Gabriela Costa Chaves – pharmacist, PhD in public health, researcher at Department of Medicines Policy and Pharmaceutical Services (NAF), Sergio Arouca National School of Public Health, Oswaldo Cruz Foundation (ENSP/Fiocruz)
The European Union (EU) is currently negotiating a free trade agreement (FTA) with the four founding members of Mercosur (Argentina, Brazil, Paraguay and Uruguay), which comprises a chapter on intellectual property rights (IPR). A new round of negotiations is taking place from November 29th to December 8th in Brussels[1]. Word is that they aim to announce the closure of the agreement at the next World Trade Organization (WTO) Ministerial Conference that will be held from 10-13 of December in Buenos Aires and the clock is ticking to close all the chapters before that. As usual, the negotiations are taking place in secrecy, but the EU released a draft proposal of the IPR chapter in September last year, which has provided the general public some knowledge about what is been negotiated.
Throughout the many years of negotiations, the Mercosur countries have been opposing the adoption of any clauses that provide further protection for IPR than is already required in the WTO TRIPS Agreement. However, according to recent press reports[2] the EU is still pushing for those measures. It is suspected that they are deliberately delaying the issue of patents and public health to try to force an agreement at the last minute, when negotiations around other issues will already have been reached and the pressure will be high on Mercosur not to lose everything.
We conducted an impact assessment study to estimate the impact that the EU proposal for the IPR chapter could have on health policies in Brazil.[3] The study aims to present new evidence to inform the negotiations and follows the recommendation of the United Nations’ High-Level Panel (UN HLP) on Access to Medicines.[4] The findings show that the adoption of the measures proposed by the EU could put the sustainability of access to health policies in Brazil at risk, as they could sharply increase public expenditures on medicines. The report was released just before the negotiations that took place last September in Brasilia, bringing new evidence to support the Mercosur position to reject the TRIPS-plus measures proposed by the EU.
If we look at only what is related to medicines used to treat HIV and hepatitis C (which amounts to 30 medicines out of the almost 450 provided by the Brazilian public health system), the reports reveal that additional expenditures could be almost BRL 2 billion per year (about USD 640 million)! That is equivalent to the annual Brazilian public expenditure on health of 1,369,256 persons![5] If that is extrapolated to all purchases of medicines in Brazil, the additional burden brought by the FTA could result in a collapse of the Brazilian public health system, one of the few in the world that adopts a policy of universal access to healthcare.
Another recommendation of the UN HLP is that “Governments engaged in bilateral and regional trade and investment treaties should ensure that these agreements do not include provisions that interfere with their obligations to fulfil the right to health” (p. 9). The EU proposal, however, contains such provisions.
We undertook an analysis,[6] released last March, of the provisions proposed by the EU and identified mainly three TRIPS-plus provisions that are harmful for health policies. Those are:
Mandatory adoption of regional or national exhaustion of intellectual property rights (IPR)
Extension of the period of protection conferred by a patent on medicinal products and
Exclusivity of data submitted to obtain market authorisation
This report also presented preliminary calculations of the impact of one of those TRIPS-plus measures – patent term extension – on public expenditures on selected medicines in Brazil. The calculations included six medicines that could have their patent term extended under such a provision: three for HIV (darunavir, etravirine, raltegravir); two for hepatitis C (sofosbuvir, daclatasvir) and one for cancer (dasatinib). It was estimated that this extension would represent an additional expenditure of nearly USD 444 million by the Brazilian Ministry of Health (MoH), in comparison with the lowest generic international prices.
The recently released report presents the findings of a more comprehensive impact assessment of two of the TRIPS-plus provisions contained in the EU proposal: patent term extension and data exclusivity. As Brazilian law already adopts the national regime of exhaustion of IPR, the impact of that specific provision was not individually calculated. The study – using the reality of the Brazilian market as a base for calculations – applies the Intellectual Property Rights Impact Aggregate (IPRIA) Model[7] in order to estimate the impact of adopting such provisions on the public expenditures and domestic sales of medicines in Brazil.
The Model was applied only to the market segment comprised by antiretroviral (ARV) medicines indicated for the treatment of HIV and to the market segment of medicines for hepatitis C, which are both provided in the public system in Brazil. It was not applied to estimate the impact of IPR changes in the Brazilian retail pharmaceutical market as a whole.
The selection of the two case studies took into consideration the significant difference between them. In Brazil, the ARV market has been relatively stable over the past years in terms of public expenditures and included an important share of generic medicines, both imported and locally produced, mostly as a result of adoption of measures to challenge patent barriers (threat and issue of compulsory license, patent oppositions, experimental use/Bolar exception and voluntary license).
On the other hand, the hepatitis C market has been sharply increasing; it is historically almost 100% under exclusivity and the sales of national producers are only residual. In 2015, there were changes in the Therapeutic Guidelines with the incorporation of the direct-acting antivirals (DAA) sofosbuvir, daclatasvir and simeprevir. The strategies adopted to try to remove IPR barriers had not fully resulted in changes in the market as of 2016, resulting in a market in which the negative impact of IPR on public expenditures and local production can be measured in full.
The study used the scenarios method to produce prospective simulation of five different scenarios in order to estimate the impact of the inclusion of each of the above-mentioned TRIPS-plus provisions proposed by the EU both separately and together:
- Base scenario – the evolution of the market if there are no changes on IP regulations in Brazil, therefore including TRIPS-plus provisions already adopted in Brazilian law
- Alternative scenario 1 – the evolution of the market in the absence of the article 40, sole paragraph, of the current Brazilian patent law, which allows for patent term extension based on patent examination delay
- Alternative scenario 2 – the evolution of the market in the case of adoption of patent term extension as a consequence of the time necessary to obtain market authorization
- Alternative scenario 3 – the evolution of the market in the case of adoption of data exclusivity for a period of 5 and 8 years
- Alternative scenario 4 – the adoption of both data exclusivity (5 and 8 years) and patent term extension due to market authorization time lag.
The main results on what is related to the variation on public expenditures are summarised in the table below.
Chart 1. Findings related to variation in public expenditures on ARV and hepatitis C medicines in Brazil
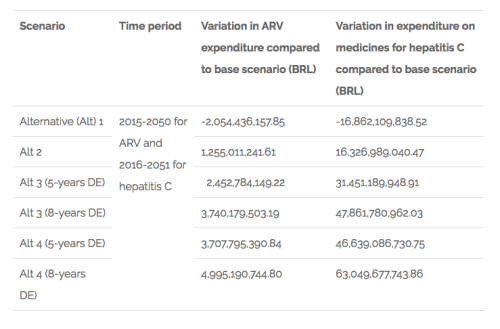
The results for what is related to the variation of sales of domestic producers in the ARV market are shown in the following chart:
Chart 2. Findings related to variation in sales of domestic producers on the ARV market in Brazil
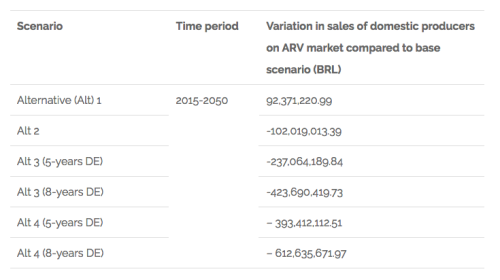
The discussion of the results highlights the implications that changing the IP law could have for policies of access to health and national development in Brazil, summarised below:
- The public expenditures on ARV in Brazil have been relatively stable in the past years as a result of multiple strategies adopted to negotiate price and remove patent barriers, such as the use of public health TRIPS flexibilities, allowing for the treatment of more people with small increase in total expenditures.
- The hepatitis C market in Brazil was almost 100% under exclusivity between 2006 to 2016. The market share of non-exclusive products in terms of sales has been residual and the fewer strategies adopted to remove patent barriers have not resulted in changes in the market yet. Public expenses have been increasing and treatment has not been available to all those in need. The impact of exclusive rights is higher in hepatitis C than in ARV as of today and will be even worse if more exclusive rights are adopted in the country.
- The adoption of the TRIPS-plus measures proposed by the EU, besides the increase in public expenditures on medicines and reduction of domestic sales shown in the study, would also reduce the policy space currently available to adopt measures to reduce the negative impact of IPR on health policies, such as the TRIPS flexibilities. That could lead to even higher increase in public expenditures and decrease of sales by national producers in the whole pharmaceutical market.
- The removal of already existing TRIPS-plus provision that extends the market exclusivity due to patent term extension would lead to savings of public money and increase in domestic sales.
- Public expenditures on medicines have been increasing in the past years, consuming rising shares of the total public health budget as a result of incorporating medicines under market exclusivity. Therefore, the adoption of new measures that increase market exclusivity is detrimental to the sustainability of the public health system.
Based on the results and discussions of the study, the authors make the following recommendations:
- The rejection of any TRIPS-plus provision that extends market exclusivity as proposed by the European Union in the negotiation of the Free Trade Agreement with Mercosur, considering the negative impact of those measures on policies of access to health and national development in Brazil.
- For the Brazilian government and other countries involved in the negotiation of the FTA to conduct a full impact study in the field of public health and human rights, as recommended recently by the UN High-Level Panel on Access to Medicines. The impact studies should be conducted transparently and be made publicly available.
- The negotiations of the FTA should be transparent and all draft texts and proposals from all parties involved should be publicly disclosed and public consultations should be held to allow the participation of all sectors of society.
- For the Brazilian government to make all efforts necessary to exclude TRIPS-plus measures already foreseen in national IP legislation, especially the removal of the provision included in the sole paragraph of article 40 of the patent law that allows for patent term extension due to delay in patent examination.
The full study is available at:
End Notes
[1] European Commission, http://trade.ec.europa.eu/doclib/press/index.cfm?id=1761.
[2] Valor Economico, October 3, 2017. http://www.valor.com.br/brasil/5142476/negociacao-frustrada-na-propriedade-intelectual
[3] Full report available in English at – http://www.ensp.fiocruz.br/portal-ensp/informe/site/arquivos/anexos/01abfe4ae54f0d6efd743fe6eea6abe259bdb702.PDF.
[4] UN SG HLP on Access to Medicines, Final Report, September 2016. Available at – http://www.unsgaccessmeds.org/final-report/.
[5] The Brazilian annual public expenditure on health on 2014 was of BRL 1,419.85 (USD 604.20) per person, according to a study published by the Conselho Federal de Medicina (CFM). Available at – https://portal.cfm.org.br/index.php?option=com_content&view=article&id=25985:2016-02-18-12-31-38&catid=3
[6] What To Watch Out For In The EU-Mercosur FTA Negotiations: Consequences For Access To Medicines. IPWatch. 22/03/2017. Available at – https://www.ip-watch.org/2017/03/22/watch-eu-mercosur-fta-negotiations-consequences-access-medicines/
The full report is available at: http://bit.ly/ftaeumercosur1 (in Portuguese) and http://bit.ly/ftaeumercosur1eng (in English).
[7] Guide to the IPRIA (Intellectual Property Rights Impact Aggregate) Model (2009). Available at –https://www.ictsd.org/sites/default/files/event/2010/03/guide-to-the-ipria-model.pdf